Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles
Wiktor Wolak
,Mirosław Roman Dudek
,Dinh Quang Ho
,Karol Marcjan
Journal of Applied Mathematics and Computational Mechanics |
 Download Full Text |
 View in HTML format |
 Export citation |
@article{Wolak_2016,
doi = {10.17512/jamcm.2016.1.19},
url = {https://doi.org/10.17512/jamcm.2016.1.19},
year = 2016,
publisher = {The Publishing Office of Czestochowa University of Technology},
volume = {15},
number = {1},
pages = {191--196},
author = {Wiktor Wolak and Mirosław Roman Dudek and Dinh Quang Ho and Karol Marcjan},
title = {Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles},
journal = {Journal of Applied Mathematics and Computational Mechanics}
}TY - JOUR DO - 10.17512/jamcm.2016.1.19 UR - https://doi.org/10.17512/jamcm.2016.1.19 TI - Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles T2 - Journal of Applied Mathematics and Computational Mechanics JA - J Appl Math Comput Mech AU - Wolak, Wiktor AU - Dudek, Mirosław Roman AU - Ho, Dinh Quang AU - Marcjan, Karol PY - 2016 PB - The Publishing Office of Czestochowa University of Technology SP - 191 EP - 196 IS - 1 VL - 15 SN - 2299-9965 SN - 2353-0588 ER -
Wolak, W., Dudek, M., Ho, D., & Marcjan, K. (2016). Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles. Journal of Applied Mathematics and Computational Mechanics, 15(1), 191-196. doi:10.17512/jamcm.2016.1.19
Wolak, W., Dudek, M., Ho, D. & Marcjan, K., 2016. Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles. Journal of Applied Mathematics and Computational Mechanics, 15(1), pp.191-196. Available at: https://doi.org/10.17512/jamcm.2016.1.19
[1]W. Wolak, M. Dudek, D. Ho and K. Marcjan, "Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles," Journal of Applied Mathematics and Computational Mechanics, vol. 15, no. 1, pp. 191-196, 2016.
Wolak, Wiktor, Mirosław Roman Dudek, Dinh Quang Ho, and Karol Marcjan. "Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles." Journal of Applied Mathematics and Computational Mechanics 15.1 (2016): 191-196. CrossRef. Web.
1. Wolak W, Dudek M, Ho D, Marcjan K. Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles. Journal of Applied Mathematics and Computational Mechanics. The Publishing Office of Czestochowa University of Technology; 2016;15(1):191-196. Available from: https://doi.org/10.17512/jamcm.2016.1.19
Wolak, Wiktor, Mirosław Roman Dudek, Dinh Quang Ho, and Karol Marcjan. "Understanding of the role of pH in filling mezoporous silica with magnetic nanoparticles." Journal of Applied Mathematics and Computational Mechanics 15, no. 1 (2016): 191-196. doi:10.17512/jamcm.2016.1.19
UNDERSTANDING OF THE ROLE OF PH IN FILLING MESOPOROUS SILICA WITH MAGNETIC NANOPARTICLES
Wiktor Wolak 1, Mirosław Roman Dudek 1, Dinh Quang Ho 1,2, Karol Marcjan 1
1
Institute of Physics, University of Zielona Gora
Zielona Góra, Poland
2 Vinh University
Vinh city, Nghe An, Vietnam
20000621@stud.uz.zgora.pl, M.Dudek@if.uz.zgora.pl
hodinhquangdhv@gmail.com, karol.marcjan@gmail.com
Abstract. Computer simulation study of filling pores of mesoporous silica with magnetic iron oxide nanoparticles which are diffusing towards the silica surface from a water solution with a given pH was presented. Three different values of the pH of the solution were under consideration, pH = 4, 7, and 10. The size of nanoparticles was of the order of magnitude of the nanopore diameter. It was observed that in the case of a low concentration of magnetic nanoparticles in the water solution the process of filling the silica pores weakly depends on the pH of the solution. The value of pH becomes only relevant for large concentration of magnetic nanoparticles. In the simulation, a 2-state Potts model was used for preparing the silica matrix. Diffusion of magnetic nanoparticles was restricted to lattice sites only.
Keywords: mesoporous silica, magnetic nanoparticles, point of zero charge
1. Introduction
Recently, an increasing number of engineering applications of magnetic nano-particles can be noted, especially those based on iron oxides which are non-toxic or where the toxicity is negligible. There are materials for medical applications like magnetic resonance [1] or drug release [2] but also new materials for industrial chemistry [3], or materials for environmental protection. In our recent papers [4, 5] we showed the special feature of iron oxide nanoparticles both experimentally and theoretically that when some amount of acid or base is added to an aqueous solution containing magnetic grains, then the solution close to the nanoparticles becomes, respectively, less acidic and less alkaline than it is expected. This property was suggested to be used in the design of new type of mortar with magnetic grains inside [5] achieving an increased compressive strength of mortar as well as its chemical protection against the acidic environment. In the following, we discuss the effect of the pH of water suspension of magnetic nanoparticles on filling the pores of the mesoporous silica. To this aim computer modeling methods have been used.
2. Preparing porous matrix
A series of models of porous glass were
introduced which were studied with the help of computer simulation methods,
like the molecular dynamics method presented in papers [6, 7]. In the
following, we used the Metropolis Monte Carlo method, instead. To model porous
glass, the 2-state Potts model on cubic lattice ![]() was chosen to simulate the phase separation of
a binary mixture by analogy to
a molecular dynamics approach presented in papers [6, 7]. The
following interaction hamiltonian between two kinds of model atoms A and
B of the binary mixture is assumed:
was chosen to simulate the phase separation of
a binary mixture by analogy to
a molecular dynamics approach presented in papers [6, 7]. The
following interaction hamiltonian between two kinds of model atoms A and
B of the binary mixture is assumed:
| (1) |
where lattice sites ![]() ,
, ![]() are occupied by atom
are occupied by atom ![]() (A or B), the angular brackets denote
summation over the nearest-neighbour lattice pairs. In the following, atoms A
represent silica material and atoms B represent vacancies. Hamiltonian in Eq.
(1) is the particular case of the hamiltonian which was introduced in paper [8]
to model the sintering process in metal powders. We assume that
(A or B), the angular brackets denote
summation over the nearest-neighbour lattice pairs. In the following, atoms A
represent silica material and atoms B represent vacancies. Hamiltonian in Eq.
(1) is the particular case of the hamiltonian which was introduced in paper [8]
to model the sintering process in metal powders. We assume that ![]() and
and ![]() .
Initially, atoms A and vacancies B are randomly placed in the sites of a cubic
.
Initially, atoms A and vacancies B are randomly placed in the sites of a cubic ![]() lattice with a given concentration
lattice with a given concentration ![]() of atoms A and
of atoms A and ![]() of
vacan-
cies, and
of
vacan-
cies, and ![]() . During the computer simulation, atoms
and vacancies which are located in the neighbouring lattice sites i, j
can exchange according
to Metropolis rule with a success rate equal to
. During the computer simulation, atoms
and vacancies which are located in the neighbouring lattice sites i, j
can exchange according
to Metropolis rule with a success rate equal to ![]() ,
where
,
where ![]() denotes the energy difference between
the new and old
lattice site configuration. In Figure 1, it has been shown the result of Monte
Carlo simulations of a cube with dimension
denotes the energy difference between
the new and old
lattice site configuration. In Figure 1, it has been shown the result of Monte
Carlo simulations of a cube with dimension ![]() lattice sites in x, y, z direction.
The periodic boundary condition was applied.
lattice sites in x, y, z direction.
The periodic boundary condition was applied.
Fig. 1. Result
of N = 600 MC steps of computer simulation of binary alloy of initially
randomly distributed atoms A and B on cubic ![]() lattice.
The parameters
which have been chosen:
lattice.
The parameters
which have been chosen: ![]() ,
,
![]() ,
, ![]() ,
, ![]() K, concentration
of A atoms
K, concentration
of A atoms ![]() . Atoms A (in red) represent model of
porous silica
. Atoms A (in red) represent model of
porous silica
3. Magnetic nanoparticles suspension
In the model, magnetic nanoparticles are
represented by spheres of radius ![]() which can occupy cubic lattice sites. The cubic
lattice is partitioned into two parts where the first one is represented by
silica sites and the remaining sites represent
a solution contacting the silica surface. Each sphere representing magnetic
nano-particle possesses magnetic dipole moment
which can occupy cubic lattice sites. The cubic
lattice is partitioned into two parts where the first one is represented by
silica sites and the remaining sites represent
a solution contacting the silica surface. Each sphere representing magnetic
nano-particle possesses magnetic dipole moment ![]() and a
point charge
and a
point charge ![]() in its center.
The value of charge is the same for each
sphere. It represents the electrostatic charge acquired by iron oxide
material when it is contacting a water solution with a given pH value. Then, there is electrostatic repulsion
between spheres as well as magnetic attraction due to the magnetic
dipolar interaction. The electrostatic part of the inter-
action potential is the following:
in its center.
The value of charge is the same for each
sphere. It represents the electrostatic charge acquired by iron oxide
material when it is contacting a water solution with a given pH value. Then, there is electrostatic repulsion
between spheres as well as magnetic attraction due to the magnetic
dipolar interaction. The electrostatic part of the inter-
action potential is the following:
| (2) |
where ![]() is the distance
between the centers of mass of the nanoparticles located at sites
is the distance
between the centers of mass of the nanoparticles located at sites ![]() and
and ![]() ,
, ![]() represents permittivity,
represents permittivity, ![]() is the Debye length
is the Debye length
| (3) |
![]() denotes
electron charge,
denotes
electron charge, ![]() is the Boltzmann constant,
is the Boltzmann constant, ![]() is the Avogadro number,
is the Avogadro number, ![]() represents ionic strength. It is
assumed that the electrostatic surface
potential of the nanoparticle can be approximated by Nernst potential, which
depends on pH as follows [11]:
represents ionic strength. It is
assumed that the electrostatic surface
potential of the nanoparticle can be approximated by Nernst potential, which
depends on pH as follows [11]:
| (4) |
![]() where is
the gas constant,
where is
the gas constant, ![]() is
the Faraday constant and PZC represents the point of zero charge. In the
following, we have chosen PZC = 7.9 for magnetic nanoparticles
and PZC = 2 for porous matrix representing silica. The surface
charge density is expressed as the following:
is
the Faraday constant and PZC represents the point of zero charge. In the
following, we have chosen PZC = 7.9 for magnetic nanoparticles
and PZC = 2 for porous matrix representing silica. The surface
charge density is expressed as the following:
| (5) |
and it is assumed that ![]() .
.
Magnetic interaction potential represents magnetic dipole-dipole interaction accord- ing to the formula:
| (6) |
where ![]() and
and ![]() represent magnetic moments of
nanoparticles at site
represent magnetic moments of
nanoparticles at site ![]() and
and
![]() ,
respectively. The interaction potentials are complemented by the Lennard-Jones
interaction potential
,
respectively. The interaction potentials are complemented by the Lennard-Jones
interaction potential ![]() which represents the short-ranged
Van der Waals interaction, respectively the magnetic nanoparticle-magnetic
nanoparticle and magnetic nanoparticle-silica in the water solution. In
general, the silica surface will acquire charge
which represents the short-ranged
Van der Waals interaction, respectively the magnetic nanoparticle-magnetic
nanoparticle and magnetic nanoparticle-silica in the water solution. In
general, the silica surface will acquire charge ![]() after
contacting water solution with a given pH which is different from the value of
after
contacting water solution with a given pH which is different from the value of ![]() . In this case, the electrostatic
interaction potential in Eq. (2) is changed to the following one:
. In this case, the electrostatic
interaction potential in Eq. (2) is changed to the following one:
| (7) |
In the model, magnetic nanoparticles in a water
suspension change the orientation of the magnetic moment and jump over
unoccupied cubic lattice sites according to the Metropolis algorithm where the
energy term in ![]() includes
all the interaction potentials. Some
fraction of magnetic nanoparticles penetrates the pores of the silica
surface and sticks to the surface. The concentration of nanoparticles in the
solution was chosen to be equal to
includes
all the interaction potentials. Some
fraction of magnetic nanoparticles penetrates the pores of the silica
surface and sticks to the surface. The concentration of nanoparticles in the
solution was chosen to be equal to ![]() to prevent agglomeration events in the solution.
The diameter of pores was of the order of
magnitude of the dimension of nanoparticle.
to prevent agglomeration events in the solution.
The diameter of pores was of the order of
magnitude of the dimension of nanoparticle.
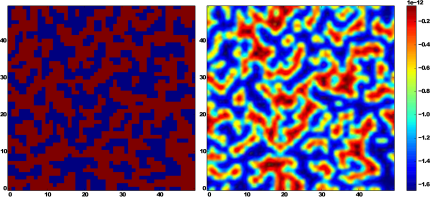
Fig. 2. On the left,
a two-dimensional projection of the simulated silica surface is shown. Red and
blue rectangles represent silica and pores respectively. On the right,
the corresponding effective interaction potential (electrostatic and Van der
Waals)
is presented which is generated by the silica and felt by nanoparticle with
radius
![]() nm in a solution with
pH = 10 at distance
nm in a solution with
pH = 10 at distance ![]() nm from the silica surface
nm from the silica surface
4. Results and discussion
Magnetic
nanoparticles which are close to silica surface experience pH-dependent electrostatic potential which strongly depends on the distance to
the surface.
In Figure 2, it was shown both the two-dimensional projection of the silica
surface (left panel) and the corresponding effective interaction potential (the
sum of electrostatic interaction potential and Van der Waals interaction
potential) of the simulated silica at ![]() at
the distance
at
the distance ![]() nm (right
panel) which is experienced by a single magnetic nanoparticle with diameter of
5 nm. The question arises to what extent the pH value affects the pore filling
in the case when the size of
the nanoparticle is comparable to the pore diameter.
For such a value of pH
as in Figure 2, magnetic nanoparticles experience strong electrostatic
repulsion but still the Van der Waals attraction is a few orders higher than
the electrostatic one. Our computer simulation suggests that the process of
filling the silica pores by magnetic nanoparticles with dimension of the order
of magnitude of the pore
diameter does not depend on the pH value when the concentration of
nanoparticles in the solution is low. Consequently, the density of
nanoparticles in silica nano-pores weakly depends on the value of pH. It is
evident in Figure 3, where the distribution of magnetic nanoparticles in the
region close to the surface of the porous matrix has been shown for three
values of pH (4, 7, and 10).
nm (right
panel) which is experienced by a single magnetic nanoparticle with diameter of
5 nm. The question arises to what extent the pH value affects the pore filling
in the case when the size of
the nanoparticle is comparable to the pore diameter.
For such a value of pH
as in Figure 2, magnetic nanoparticles experience strong electrostatic
repulsion but still the Van der Waals attraction is a few orders higher than
the electrostatic one. Our computer simulation suggests that the process of
filling the silica pores by magnetic nanoparticles with dimension of the order
of magnitude of the pore
diameter does not depend on the pH value when the concentration of
nanoparticles in the solution is low. Consequently, the density of
nanoparticles in silica nano-pores weakly depends on the value of pH. It is
evident in Figure 3, where the distribution of magnetic nanoparticles in the
region close to the surface of the porous matrix has been shown for three
values of pH (4, 7, and 10).
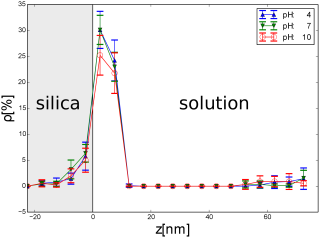
Fig. 3. Space
dependence of the density ![]() of magnetic
nanoparticles in the region close
to the surface of the porous matrix which is contacting solution with a given
value of pH is shown. All nanoparticles have a radius equal to
of magnetic
nanoparticles in the region close
to the surface of the porous matrix which is contacting solution with a given
value of pH is shown. All nanoparticles have a radius equal to ![]() nm.
Three values of pH were chosen, pH = 4, 7, and 10
nm.
Three values of pH were chosen, pH = 4, 7, and 10
There is no significant difference between the fragments of the plots corresponding to the interior of silica. The effective potential experienced by magnetic nanoparticles approaching the silica surface strongly depends on their distance to the surface. In the case of large concentration of magnetic nanoparticles, the role of the value of pH increases. In paper [10], the preferential filling the silica pores by the alkaline solution with magnetic nanoparticles was observed. The results strongly depend on the value of PZC of the materials under consideration. In particular, in the case of magnetic iron oxide nanoparticles can take the value between 6.3 [5] and 7.9 [11]. In a magnetic suspension with the large concentration of magnetic nanoparticles, the dipolar magnetic interactions become important and they compete with the pH-dependent electrostatic repulsion between the nanoparticles.
5. Conclusions
A computer simulation of the process of filling the nanopores of the silica glass by water magnetic suspension at given value of pH was under discussion. It was concluded that pH value is significant for the surface adsorption of the nanoparticles but filling the pores by them weakly depends on the value of pH in the case of low concentration of magnetic nanoparticles.
Acknowledgements
We thank Bartłomiej Zapotoczny for his discussion and comments on the work.
References
[1] Lodhia J., Mandarano G., Ferris N.J., Eu P., Cowell S.F., Development and use of iron oxide nanoparticles (Part 1): Synthesis of iron oxide nanoparticles for MRI, Biomed. Imaging and Intervention Journal 2010, 6(2), e12.
[2] Huang S., Li C., Cheng Z., Fan Y., Yang P., Zhang C., Yang K., Lin J., Magnetic Fe3O4 mesoporous silica composites for drug delivery and bioadsorption, J. Colloid. Interf. Sci. 2012, 376, 312-321.
[3] Lu A.H., Salabas E.L., Schuth F., Magnetic nanoparticles: Synthesis, protection, functionalization, and application, Angew. Chem. Int. Ed. 2007, 46, 1222-1244.
[4] Zapotoczny B., Dudek M.R., Koziol J.J., Mleczko J., Nanobuffering property of Fe3O4 magnetic nanoparticles in aqueous solution, Physica A 2013, 392, 1493-1499.
[5] Borucka-Lipska J., Kiernozycki W., Guskos N., Dudek M.R., Ho D.Q., Wolak W., Marc M., Koziol J.J., Kalaga J.K., Investigation on magnetite concentrate with respect to its use in concrete, submitted.
[6] Gelb L.D., Gubbins K.E., Characterization of porous glasses: Simulation models, adsorption isotherms, and the Brunauer-Emmett-Teller analysis method, Langmuir 1998, 14(8), 2097-2111.
[7] Gelb L.D., Gubbins K.E., Pore size distributions in porous glasses: a computer simulation study, Langmuir 1999, 15(2), 305-308.
[8] Dudek M.R., Gouyet J.F., Kolb M., Q+ 1 state Potts model of late stage sintering, Surface Science 1998, 401(2), 220-226.
[9] Keren R., Sparks D.L., Effect of pH and ionic strength on boron adsorption by pyrophyllite, Soil Science Society of America Journal 1994, 58(4), 1095-1100.
[10] Zapotoczny B., Dudek M.R., Guskos N., Kozio J.J., Padlyak B.V., Kosmider M., Rysiakiewicz- -Pasek E., FMR study of the porous silicate glasses with Fe3O4 magnetic nanoparticles fillers, Journal of Nanomaterials 2012, 8.
[11] Tombacz E., Majzik A., Horvat Z.S., Illes E., Magnetite in aqueous medium: coating its surface and surface coated with it, Romanian Reports in Physics 2006, 58(3), 281-286.


